CONCLUSIONS
Diffuse large B-cell lymphoma (DLBCL) is diagnosed at a median age of 65 years, and around 40% of the patients are older than 70 years. Because of frailty and comorbidities, standard chemoimmunotherapy delivery may be very challenging in this setting. Therefore, a more personalized approach based on comprehensive geriatric assessment (CGA) may be warranted, as already proposed by other international groups. We aim to evaluate the impact of the establishment of a CGA on therapeutic decisions and outcome in our older population of pts with DLBCL.
From January 2018 to May 2023, some unselected (depending on the referring hematologist) patients ≥70 years old with DLBCL were prospectively evaluated by a geriatrician at Catalan Institute of Oncology. CGA included validated instruments to assess: comorbidity with Charlson Index and Cumulative Illness Rating Scale (CIRS); polypharmacy; functional status in terms of activities daily living (ADL) with Barthel scale and instrumental ADL with Lawton scale; geriatric syndromes; mood with Yeasauvage scale; cognition with Pfeiffer scale; and social status. According to global CGA, patients were classified in 3 frailty groups: type 1, fit for standard chemoimmunotherapy (S-CT); type 2, fit for reduced intensity chemoimmunotherapy (RI-CT); type 3, candidate to palliative treatment.
Among 247 newly diagnosed DLBCL, 101 (41%) patients had ≥70 years, of which 38 underwent CGA. Among patients who underwent CGA, 14 (37%) were classified as type 1, 20 (53%) type 2 and 4 (10%) type 3. Type 1 patients were younger (median age 75, ≥80 yrs 29%), than type 2 (median age 79, ≥80 yrs 55%), and type 3 (median age 93, ≥80 yrs 100%). The characteristics of 63 pts ≥70 years who did not underwent CGA did not differ significantly from the population in the study, except they had lower extranodal involvement and a higher proportion of patients did not received treatment (Table 1).
All the evaluated 38 patients received treatment (Figure 1), 34 with immunochemotherapy (Table 1), and 5 patients (4 of type 3 and 1 of type 2) were palliatively handled: 1 with radiotherapy and 4 with cyclophosphamide monotherapy with or without prednisone. During treatment, 2 patients (one of type 1 and one of type 2) were deescalated from RCHOP to RminiCHOP due to severe infectious complications, while 6 patients of type 2 were upgraded, as previously planned if treatment was well tolerated. The most common adverse event was infection in 36% of patients (in 21% grade 3/4). Twelve patients (4 of type 1 and 8 of type 2) experienced at least an infectious event. Two toxic fatal events (1 patient of type 2 of septic shock and 1 of type 1 of urinary tract infections in the contest of bladder fistula) were reported. There were treatment delays in 10 (29%) patients (5 of type 1 and 5 of type 2).
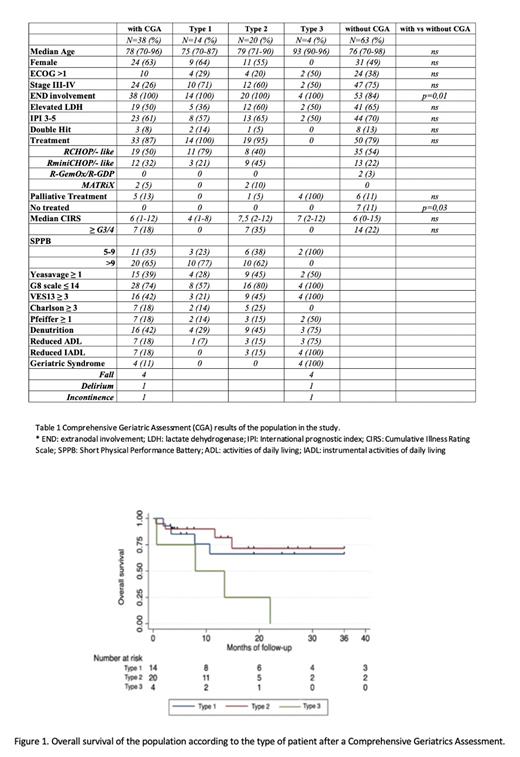
Among 33 patients who received active treatment, 4 had not been evaluated yet, and 29 were evaluable for response: overall response rate (ORR) was 71% and the complete remission (CR) rate was 68% (71% with S-CT and 68% with RI-CT). With a median follow-up of 12 months (range, 1-133), the 1-year event-free survival (EFS) and overall survival (OS) of the study population were 57% (95% CI, 38-71) and 72% (95% CI, 52-84), respectively. Among patients without CGA, the 1-year EFS and OS were 57% (95% CI, 44-69) and 67% (95% CI, 53-78), respectively. According to CGA, the 1-year EFS of type 1 patients was 68% (95% CI 35-87), type 2 was 61% (95% CI, 34-79) and type 3 was 0%. The 1-year OS of type 1 patients was 66% (95% CI 32-86), type 2 was 82% (95% CI, 52-94) and type 3 was 50% (95% CI, 1-84) (Figure 1).
In this homogeneous series of older patients with DLBCL, our results confirmed the importance of CGA. Within a multidisciplinary approach, we may accurately classify the fitness of patients and offer them the best therapeutic options without causing excessive toxicity, as proved by similar EFS estimates between type 1 and 2 subgroups. Further larger studies are needed to evaluate potential geriatric interventions aimed at further improving outcomes.
Disclosures
Domingo Domenech:BeiGene: Consultancy; Takeda: Consultancy, Honoraria, Speakers Bureau; BMS: Speakers Bureau. De Oliveira:Janssen, Alexion: Consultancy; Janssen: Other: Travel Expenses. Sureda Balari:Astra Zeneca: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Kite: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; MSD: Consultancy, Honoraria; Jannsen: Consultancy, Honoraria; GenMab: Consultancy, Honoraria; Pierre Fabre: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Gonzalez Barca:Janssen, Abbvie, Takeda, EUSAPharma, AstraZeneca, Lilly: Speakers Bureau; Janssen, Abbvie, AstraZeneca: Other: Travel; Janssen, Abbvie, Kiowa, EUSA Pharma, Beigene, Sobi: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal